Ageing is an inevitable complex process of life and consequently is described as a gradual loss of molecular fidelity of cells after reaching sexual maturity and as a result this culminates into the loss of function and sequentially leads to disease and death. For example, in most organisms, the rate of aging is inversely proportional to mean lifespan. Additionally, increase in age is also the largest risk factor for several different diseases like cancer, neuro-degeneration and cardiovascular disease.
Epigenetics is the study of changes in organisms caused by modification of gene expression rather than alteration of the genetic code itself (Rodriguez-Rodero et al., 2011, Fraga et al., 2007).
Aging in particular, is a multi-factorial process that is determined by genetic and environmental factors. Additionally, this means that different members of a species show a variation in their genotype and thus determines the lifespan. Correspondingly this suggests that the variability is more severely affected by a tendency of small molecular errors that accumulate compromising the adult stem cell function (Kirkwood, 2005).
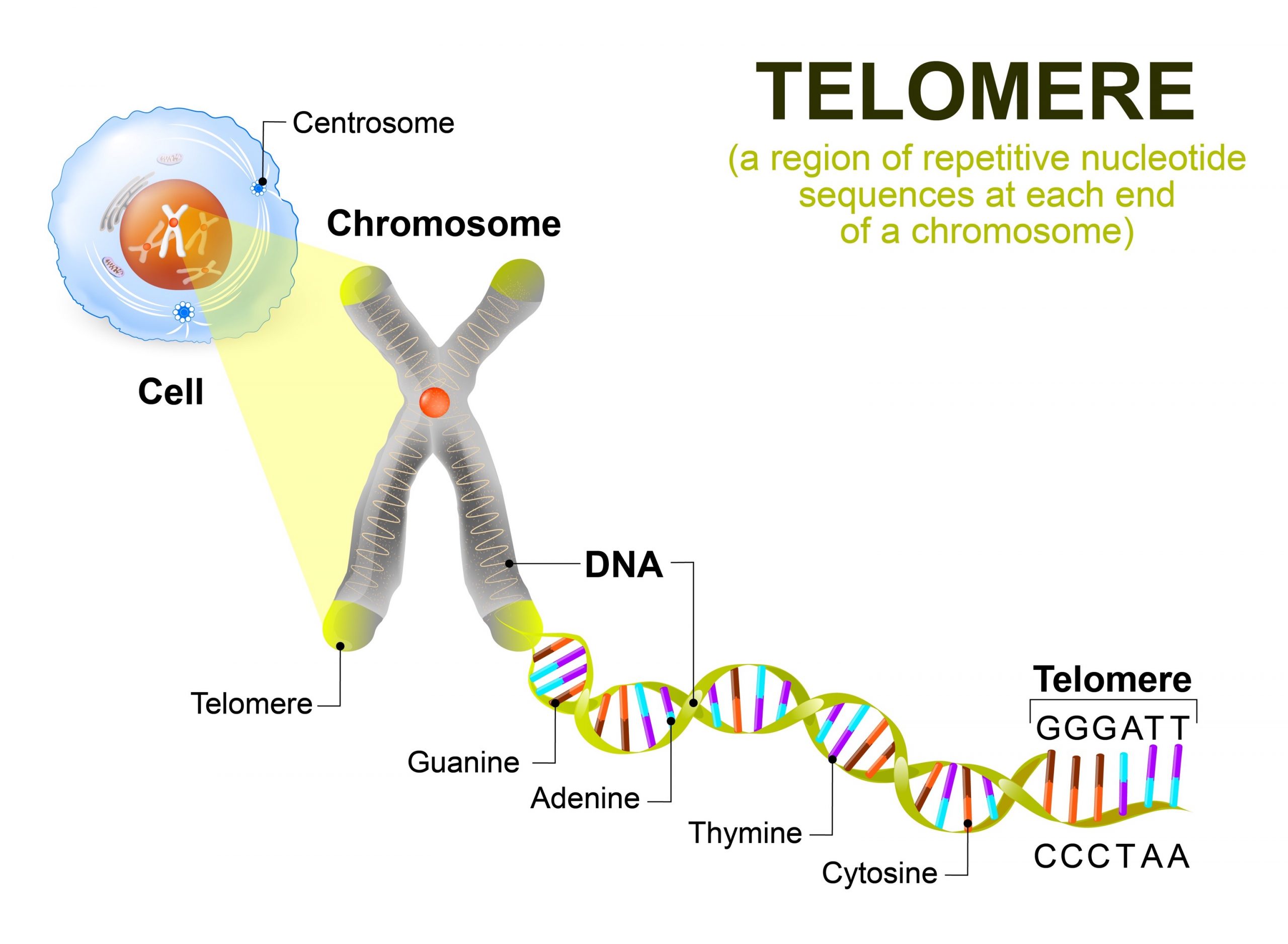
Various studies have documented an age related increase in mutations of DNA especially somatic mutations and other form of DNA damage. In particular, the absence of the enzyme telomerase has been implicated in the decline of cell division in the somatic cells since the telomeres of the chromosomes progressively shorten with each cell division (Kim Sh et al., 2002). For example, oxidative stress has also been linked to telomere loss, having been shown to have an effect on the rate of telomere loss and as a result this is linking telomere erosion with damage (von Zglinicki, 2002).
Genetics and Epigenetics Influence on Stress and Ageing
There appears to be many theories that have traditionally been put forward to try and explain the evolutionary mechanisms involved in aging. In particular, these include: the theory of programmed death, the mutation accumulation theory of aging, the antagonistic pleiotropic theory of aging, and the evolutionary maintenance theory (Moskalev et al., 2014).
The programmed cell death theory was initially proposed by Weisman et al. who argued that natural selection inheritable programmed death so as to limit the lifespan of an individual and to clear space for new generations (Weismann et al., 1891). His preposition was however shot down by Haldane, Medawar, and Williams, who suggested that aging was more of a stochastic than programmed, since the forces of natural selection diminish with adult age, more so after the peak of reproduction. Hamilton came up with a mathematical equation dubbed the “Hamilton’s forces of natural selection” that showed that indeed the forces of natural selection declined with age. This was later confirmed to be true with experiments on Drosophila (Medawar, 1946, Williams, 1957, Gavrilov and Gavrilova, 2002, Rose et al., 2008).
The mutation accumulation theory was proposed by Medawar (Medawar, 1952). Conversely this theory proposes that, although natural selection could get rid of damaging variation in early stages of the life cycle, it is less efficacious at the later stages of post reproductive life. Suggesting that mutations that have late acting effects will tend to accumulate over evolutionary time thereby resulting to an age related mortality. An additional component to this theory was added by Williams who suggested that the same late-acting deleterious effects do have an early-acting beneficial effect. Additionally, advances in molecular genetics have identified a few genes and genetic pathways which when compromised can lead to a substantial increase in life span. In particular, variations in three loci have been shown to influence survival beyond 90 years of age in various populations: the APOE gene (Schachter et al., 1994, Rea et al., 2015), the FOXO3A gene in the insulin‐IGF1 pathway (Willcox et al., 2008, Flachsbart et al., 2009, Soerensen et al., 2010), and a region of unknown function on chromosome 5q33.3 (Deelen et al., 2014). Calorific restriction has also been linked to longevity. As an example in Okinawans, caloric restriction and traditional functional food have been suggested to play a role in extension of lifespan (Willcox and Willcox, 2014).
The antagonistic pleiotropy theory was proposed by Williams (Williams, 1957). According to this theory, the declining force of selection with age results in selection for pleiotropic genes which enhance fitness early in life but reduce it later. The accumulation of many such genes could therefore result to a deterioration of condition with age (Williams, 1957). There is limited genetic evidence to support this theory. In a summary of evidence by Capri et al. they gave a strong genetic component for survival at extreme ages in humans. They showed that the genes that are positively associated with longevity appeared to belong mostly to a group of stress-response genes, which have a potential to act as modifiers of other genes which have varying metabolic roles. The metabolic genes have varying biological functions across ages, and thus antagonistic pleiotropy can have a major role in humans, where sufficient post-reproductive individuals occur for its detection (Capri et al., 2006). Antagonistic pleiotropy is however unlikely to have any consequence under environmental conditions of natural populations were restricted lifespans prohibits most survival to old age. In such circumstances, positive pleiotropy is the likely outcome (Parsons, 2007).
The accumulative waste theory of aging, also known garbage accumulation theory of aging is a theory that proposes that molecules damaged by oxidation and their byproducts such as collagen and damaged enzymes and organelles such as damaged mitochondria tend to accumulate in post mitotic cells and cause dysfunction, toxicity, aging, and cell death. The presence of waste products alters the structural organisation of the cell. The waste products displace the cellular components thus affecting the cellular functions such as cell signalling, transport of cellular molecules, and metabolic functions. The accumulation of these waste materials cause oxidative stress to the cells leading to ageing (Hayflick, 1985, Brunk and Temían, 1999). The presence of reactive oxygen species have the potential of damaging DNA especially mitochondrial DNA and this will affect the longevity of cells and consequently as a result of an organism.
Aging is associated with a decline in multiple areas of immune function (Burns and Goodwin, 1997). Aging is associated with increased autoimmunity and inflammation coexistent with a state of immunodeficiency (Sardi et al., 2011). In 1969, Walford put forward the auto-immune theory (Walford, 1969). According to this theory, with progression of age, the immune system tends to lose efficiency and experiences widespread dysfunction leading to widespread autoimmunity (Diggs, 2008). There appears to be two age related processes that are linked to causation of autoimmune diseases: different rates of senescent cell accumulation in the immune system and target tissue/organ and the heterogeneous accumulation of senescent cells in tissues/organs (Pietilä et al., 2015). These two processes either separately or combined are at the base of autoimmune diseases (Manestar-Blazic and Volf, 2009). The primary cause of auto immunity is the involution of the thymus with a decline of naive T cells with the production of autoantibodies being secondary (Prelog, 2006). An increase in the levels of CD5+ B lymphocytes in the elderly population is instrumental in autoimmunity as these cells are responsible for production of autoantibodies and lead to an imbalance of the mechanism controlling the immune response against self-antigens (Buffa et al., 2011, Weksler, 2000). There is a close link between autoimmune responses during aging and epigenetics. While epigenetic changes in aging result in largely unpredictable changes in gene expression, some of these changes represent unidirectional adaptive changes that contribute to the basic biological underpinning of aging (Pal and Tyler, 2016).
Although general DNA hypomethylation generally occurs during aging, progressive age-dependent loss of DNA methylation occurs at specific gene promoters, including ITGAL and IL17RC, which lead to their transcriptional induction and subsequent autoimmune responses (Wei et al., 2012). T cells are mostly affected during the aging process and a recent study revealed hyper methylated sites primarily located at CpG islands of silent genes and enriched for repressive histone marks of CD8 cells from older individuals (Tserel et al., 2015).
The cross-linking theory of aging was proposed in 1942 by Johan Bjorksten (Bjorksten, 1968). According to this theory, when cross-linked proteins accumulate, they damage cells and tissues, slowing down bodily processes and this results to aging. This has been supported by recent studies which have shown that cross-linking reactions are involved in the age related changes in the proteins that were studied (Bjorksten and Tenhu, 1990).
Gerschman introduced the free radical theory in 1954, although it had previously been developed by Denham Harman (Gerschman et al., 1954, Harman, 1956). This theory proposes that superoxide and other free radicals cause damage to the macromolecular components of the living cell, giving rise to accumulated damage causing cells, and eventually organs, to stop functioning. The macromolecules such as nucleic acids, lipids, sugars, and proteins are susceptible to free radical attack. Nucleic acids can get additional base or sugar group; break in a single- and double-strand fashion in the backbone and cross link to other molecules. Although the body has some natural antioxidants in the form of enzymes, which help to curb the dangerous build-up of these free radicals, these tend to decrease with age (Afanas’ev, 2010).
The Somatic DNA damage theory proposes that DNA damages occur continuously in cells of living organisms. Naturally, some of the damages are repaired while others accumulate, since the DNA polymerase and other repair mechanisms are not capable of correcting all of the damages as fast as they are apparently produced. In particular, there is evidence for DNA damage accumulation in non-dividing cells of mammals. Genetic mutations occur and accumulate with increasing age, causing cells to deteriorate and malfunction. In particular, damage to mitochondrial DNA might lead to mitochondrial dysfunction. Therefore, aging results from damage to the genetic integrity of the body’s cells (Kenyon, 2010).
The accumulation of damage and loss of mitochondrial genome integrity is hypothesised to play a central role in the aging process. One consequence of oxidative phosphorylation in mitochondria is that electrons escape the electron transport chain and form reactive oxygen species (ROS). The free radicals generated are responsible for damaging different cellular components such as proteins, lipids, and DNA. The proximity of mtDNA to the electron transport chain and its lack of protective histones renders mtDNA especially vulnerable to ROS-mediated damage. Since the DNA repair in the mitochondria is not as robust as the nuclear genome, mtDNA is believed to be highly susceptible to mutagenesis. The gradual accumulation of mutations to mtDNA over time results in mutant electron transport proteins encoded in the mtDNA that, in turn, leads to the release of more electrons and increased ROS production. Thus, a ‘vicious cycle’ of ROS damage-induced ROS production may play a critical role in the aging process (Harman, 1956, Harman, 1972).
Conclusion
Aging is a complex process that is best described as a group of cellular functions that participate in an integrated way in the process of senescence. The great variability in longevity between individuals of the same species suggests that the aging process is profoundly affected by processes that lead to the accumulation of errors that damage repair systems and compromise stem cell function. These changes can be caused through genetic and epigenetic mechanisms, which are influenced by genes, environmental and stochastic factors whose contribution remains to be determined by future studies.
About the Author:
Based in Perth, Western Australia, Christine Comans is a Clinical Aesthetician, trainer and educator and author who specialises in intradermal micro-pigmentation for women after mastectomy and breast reconstruction surgery. Chris holds a degree in Applied Health Science (Clinical Aesthetics) she is a strong advocate for industry standards and is renowned for her love of learning and sharing her knowledge.





13 Responses