REGISTRATION, EVALUATION, AUTHORISATION AND RESTRICTION OF CHEMICALS
European Society of Tattoo Pigment and Research - ESTP & Research
This year I am involved in a research project that is investigating the affects of carbon black tattoo inks in the body. Last year as I completed my Graduate Certificate in Medicine – Skin Cancer at the University of Queensland, I was granted international membership – clinical specialists and researchers with merits, non-Europeans to the European Society of Tattoo Pigment and Research ESTPR. This is exciting research and I will update industry as I progress.
The REACH pigment legislation comes into effect 04th January 2022.
REACH stands for Registration, Evaluation, Authorisation, and Restriction of CHemicals.
These regulations require any company producing, importing, using or placing a substance, mixture or an article on the EU market is responsible for making sure they are compliant with the REACH Regulation. The legislation covers not only European producers, including chemical distributors, suppliers, and downstream users but also any EU companies importing products to the EU market.
Approximately 4130 substances are subject of the Registration, Evaluation, Authorisation and Restriction of Chemicals; REACH regulation of tattoo inks. Using substitutable pigments and effective and safe preservatives along with a selection of auxiliary compounds shouldn’t be too difficult to achieve. It just means changing the way things have
traditionally been manufactured. For most of us working within the industry these changes should be embraced.
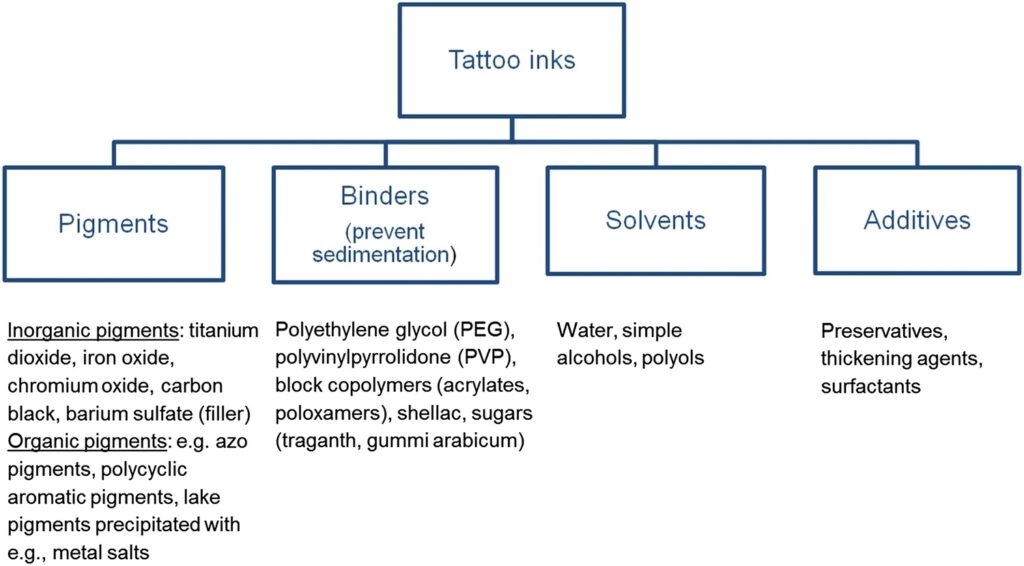
Pigments, Binders, solvents and additives are bound by reach
What does it mean for cosmetic tattooists?
Inks or other mixtures used for tattooing are absorbed into the body. Mixtures used for tattooing generally consist of colorants and auxiliary ingredients such as solvents, stabilisers, wetting agents, pH regulators, emollients, preservatives and thickeners. The mixtures are introduced into the human skin, inside the eyeball or into mucous membranes. The colorants mostly remain close to where the mixture is administered, so that the tattoo or permanent make-up will remain visible. However, the soluble ingredients in the mixture are distributed within a matter of hours or days across the entire body. In consequence, the skin and other organs are exposed to the effects of those soluble substances over an extended period. Some of those substances have hazardous properties that pose a potential risk to human health. In addition, metabolism of the colorants in the skin, decomposition due to solar radiation exposure and laser irradiation may also lead to the release of hazardous chemicals from the area of the body where the tattoo or permanent make-up is located, frequently found in lymph nodes and the liver.
Risks to human health
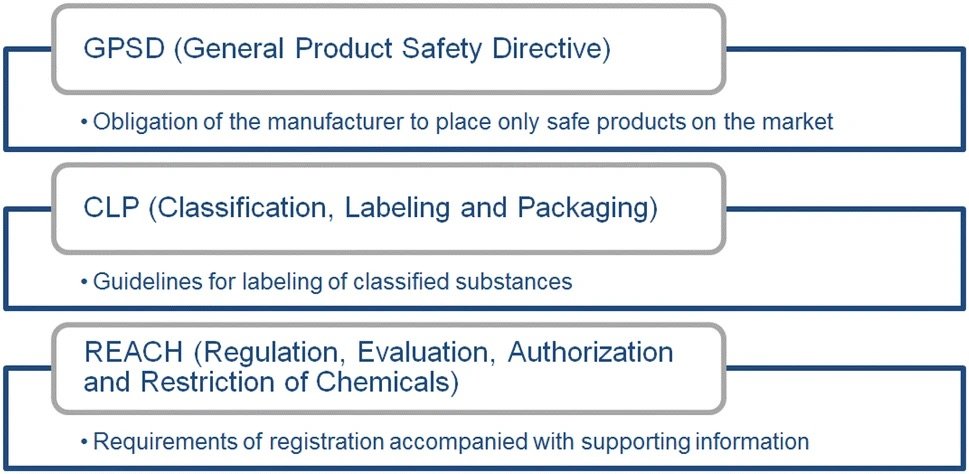
Risks to human health due to exposure to certain hazardous chemicals in mixtures used for tattooing purposes are not adequately controlled and need to be addressed to achieve a harmonised high level of protection to human health. The new restrictions include prohibiting both the placing on the market of mixtures for use for tattooing purposes and the use of mixtures for tattooing purposes if they contained any substances that contain carcinogenicity, mutagenicity or toxicity to reproduction, skin sensitisation, skin corrosion, or skin irritation, or serious eye damage or eye irritation ingredients listed in the relevant categories. This means it’s not just the colourants it also includes the preservatives. Preservatives, a substance group with specific functional properties, may pose human health risks, as well. Adverse effects appearing directly after tattooing or years later have been monitored in dermatological clinics and are comprehensively summarized by Kluger (2019) and Serup et al. (2016).Further directives are set out for the manufacturers as shown in the diagram below.
Labelling Requirements
Labelling requirements are to state the fact that the mixture is for tattooing purposes, a requirement to specify a unique reference number for identifying the specific batch, a requirement to list any ingredients classified as hazardous to human health (but not covered by the proposed restriction, and any ingredients covered by the proposed restriction but used in the mixture below the concentration limit set by the proposed restriction). Furthermore, an additional labelling requirement to indicate the presence of nickel and chromium was considered necessary, as these particular substances can induce new cases of skin sensitisation and potentially trigger allergic reactions in sensitised persons. The labelling requirements were proposed in order to give consumers and tattooists additional information, to facilitate implementation of the restriction, and to ensure that investigations can be properly carried out in the event of adverse health effects.
Cosmetic product regulation
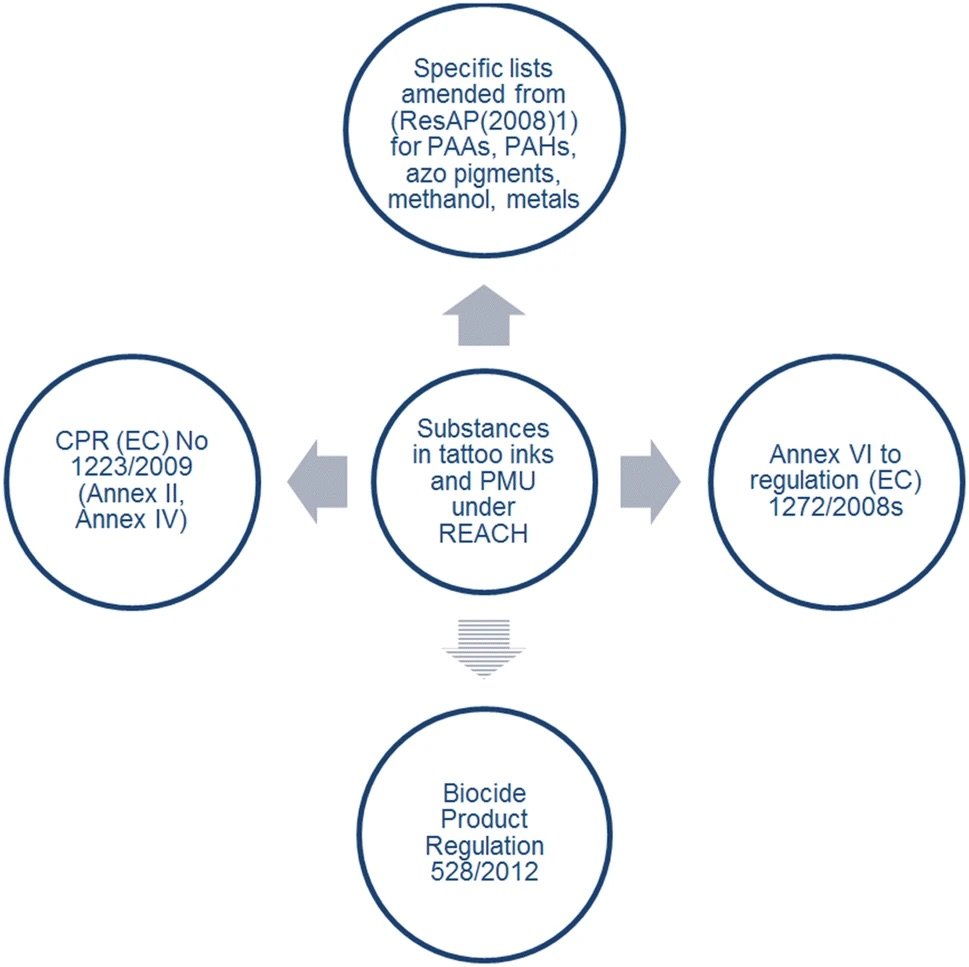
REACH incorporates regulatory annexures as shown in the diagram below. A major number of substances are regulated through the Cosmetic Product Regulation (CPR) (EC) No. 1223/2009. Under the assumption that substances prohibited in cosmetic products are at least as hazardous when injected into the skin, they shall be prohibited in tattoos and PMU, as well. Annex II of CPR (EC) 1223/2009 includes the list of banned substances, while Annex IV sets conditions and concentration limits for colorants allowed in cosmetic products. With regard to the use of preservatives, a Norwegian positive list for preservatives exists, and it comprises 21 substances. These examples indicate that most of the substances considered by the REACH restriction are not being used in tattoo inks.
Product Labelling
To facilitate compliance of tattooists with this restriction, only mixtures that are marked with the statement ‘Mixture for use in tattoos or permanent make-up’ should be used for tattooing purposes. That sounds simple enough! All the best to my fellow cosmetic tattooists
