Lymphatic drainage of the breast
Functional Drainage of the Breast
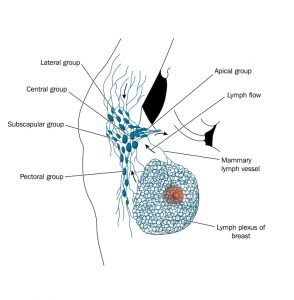
Approximately 75-90% of the breasts’ lymphatic drainage is to the same side (ipsilateral) axillary nodes. The majority of the breast lymphatics drain into the axillae along a plane subdermal while collecting at the lateral border of the pectoralis major muscle in a single sentinel node.
The superficial lymphatics of the areola and the nipple all collect in a heavy network of pre-collectors commonly known as the Sappey subareolar plexus. The breast parenchyma lymphatics originate within the walls of the lactiferous ducts and in the interlobular tissue. A portion of the deep breast tissue, in particular, the medial breast, collects into lymphatic vessels that puncture the deep fascia to drain to the internal mammary group of lymph nodes. Some of the lymphatics may pass within the breast parenchyma through the tiny interval nodes contained within it. Periodic drainage into the supraclavicular, contralateral internal mammary nodes, subclavicular, or the subscapular nodes has also been found to occur.
Axillary Lymph Nodes
The axillary lymph nodes are a group of lymph nodes that are found in the axillary region of the upper limb. The axillary region is otherwise known as the ‘armpit’ even though it is structurally a three-dimensional space that is bound by the clavicle interiorly and the skin inferiorly. The axillary region is quadrilateral space that changes its shape based on the movements of the upper limb, such as adduction and abduction. There are approximately 20 to 30 separate nodes that are found within the axillary region.
The axillary lymph nodes are chiefly responsible for the lymphatic drainage of the majority of the human body and therefore have significant clinical relevance. The clinical relevance is revealed by the nodal status in breast cancer, which helps in the determination of the treatment approach and algorithm. The axillary nodes drain the vessels of the upper limb, abdomen above the umbilicus, lateral quadrant of the breast, and the vessels of the upper limb, among other regions.
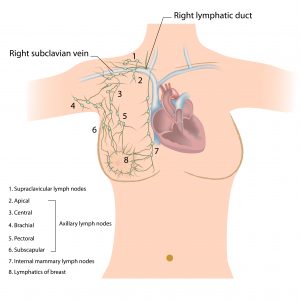
The axillary lymph nodes are commonly divided into six groups:
The Apical Axillary Group
The apical group of axillary lymph nodes is also known as the subclavicular group. This group of nodes is composed of 8-12 nodes that lie between the superior border of the clavicle and the pectoralis minor muscle, and to the first rib laterally. The apical group of axillary lymph nodes receives its drainage from the rest of the levels and groups of the axillary lymph nodes and drain mainly to the subclavian trunk. The subclavian trunk flows to the thoracic duct on the right and left lymphatic trunk on the right side of the human body.
The Brachial Group
The brachial group of axillary nodes are otherwise known as the lateral of the axillary group of nodes and are comprised of 4-6 nodes posterior and medial to the axillary vein. This group of lymph nodes receive a major share of drainage from the upper torso and drain into the apical group of the axillary lymph nodes.
The Central Group
The central group of axillary lymph nodes lye within the axillary adipose tissue and deep into the pectoralis minor muscle. This group is composed of about 4-5 nodes that receive drainage from the brachial group, the subscapular group, the pectoral group, and the breast.
The Subscapular Group
Otherwise known as the scapular or posterior group of axillary nodes, the subscapular group is composed of about 5-7 nodes located anteriorly to the subscapularis muscle and on the lateral border of the scapula.
The Interpectoral Group
The interpectoral group of axillary nodes are otherwise known as Rotter’s nodes and typically consist of 1-4 nodes lying between the pectoralis minor and major muscles. This group of axillary lymph nodes get their lymphatic drainage from the breast directly and drain into the pectoral and apical axillary group of nodes.
The Pectoral Group
The pectoral group of nodes is also known as the external mammary group or the anterior group. This group of nodes consists of about 5-6 nodes that are found along the lateral thoracic vessels inferiorly to the pectoralis minor muscle, and superiorly to the border of the pectoralis major muscle. The pectoral group of axillary lymph nodes receive its drainage majorly from the lateral aspect of the abdominal wall and the breast and drains into the central group of axillary lymph nodes.
The Infraclavicular group
Otherwise known as the deltopectoral group, this group of nodes to not typically form part of the axillary chain and levels of lymph nodes. This group of nodes le between the borders of the deltoid muscle, pectoralis major muscles, and the clavicle. The group is surrounded by the cephalic vein, and drainage is mainly received from the hand and forearm.
The Internal mammary nodes
The internal mammary group of nodes is also referred to as the parasternal group. This group of nodes travels along the internal mammary vein and artery and within the intercostal spaces and to the parietal pleura deeply. The perforating branches of the internal mammary artery are accompanied by perforating lymphatics and extrude through the pectoralis muscles and the deep fascia. There is a general variation in breast supply via the perforators, which clearly explains the risk of breast cancer to metastasize in all breast quadrants via the internal mammary lymphatics, mostly in the deep medial aspect of the breast.
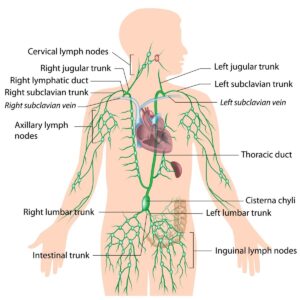
Thoracic Duct
The thoracic duct is considered as the chief lymphatic duct in the body. The thoracic duct transports lymph from the abdomen, lower limb, face, neck, and left the side of the trunk. The thoracic duct measures approximately 40 cm in length and 3-5 millimeters in diameter. The thoracic duct drains into the junction of the left subclavian vein and left jugular vein. The lymphatic drainage of the body and bowels enters systemic circulation at this junction. The drainage of the right upper trunk, face head and neck is to the right lymphatic duct.
Breast cancer
Breast cancer is a menace globally. Statistics indicate that breast cancer affects 1 in every eight women in their lifetime. Approximately over 250,000 new cases of breast cancer are diagnosed, and over 40,000 deaths are linked to breast cancer annually in the US. Early detection by utilizing cancer screening combined with advances in the treatment modalities have significantly improved the 5-year survival rates to 90%.
In women with localized cancer that does not involve the lymph nodes, the estimated 5-year survival rate is estimated at 99%. The survival rates are estimated at 85% in cases of lymph node involvement, whereas the survival rates drop drastically to 27% in cases of distant metastases. The incidence of breast cancer in men is less common, with about 1% of breast cancers being diagnosed among men in developed countries. The prognosis among bother gender is similar, although male cancers typically present at later stages.
Risk factors
The common risk factors for breast cancer include late menopause, use of hormone replacement therapy, early menarche, and nulliparity due to an increased estrogen exposure risk. Among the high risks for breast cancer also include a positive family history for ovarian cancer or breast cancer, especially in patients who present with a genetic mutation of the BRCA gene. It is estimated that women with gene mutations of BRCA1 have a high risk of about 60% of developing breast cancer by age 70. The reduction of about 25% in mortality rates attributed to breast cancer can be linked to the aggressive routine mammography screening for women aged 50 and above in countries with screening programs.
A family history of breast cancer is a significant risk factor for the development of breast cancer. It is estimated that approximately 20% of women that develop breast cancer in their lifetime have a first degree relative with a history of breast cancer. Among women with a positive family history for breast cancer, about 20% of them have a germline mutation, which has been shown to increase the susceptibility to breast cancer as well as other malignancy forms, but mostly in those with BRCA1 or BRCA2 mutation genetic mutation. Genetic screening and counseling is highly recommended in women who are diagnosed with breast cancer at the age of 50 or less. Genetic counseling should also be advised for women who develop more than one primary cancer, those with invasive ovarian tumors and men who are diagnosed with breast cancer.
Diagnosis
The diagnosis of breast cancer is through the detection of abnormal results of a mammogram in countries that have screening programs such as mammography. The identifications of radiologic findings such as microcalcifications within the soft breast tissue are highly suggestive of invasive breast cancer. Classically, breast cancer may also present with a breast mass that is singular, firm, immobile, and irregular. Physical examination through palpation may reveal palpable lymph nodes, retraction of nipple, and skin changes, which are highly suggestive of the cancer being in advanced stages. To make a definitive diagnosis, histological examination obtained through core needle biopsy, surgical removal, or fine-needle aspiration should be utilized.
Studies show that approximately 25% of breast neoplasms present typically as ductal carcinoma in situ (DCIS), where the breast malignancy is only limited to the ducts of the breast. However, there is a risk for this form of the neoplasm to progress to infiltrating ductal carcinoma (IDC), which is identified as the most common form of breast cancer. Therefore, in case ductal carcinoma in situ (DCIS) is suspected, it should be treated aggressively. Of the remaining 30% of invasive neoplasms, the presentation is either mixed ductal/lobular, carcinoma invasive lobular carcinoma and rare papillary, inflammatory, mucinous, Paget’s disease, among other forms. The prognosis and selection of therapy and treatment is highly dependent on the grade of the tumor, the molecular subtype, and the hormonal status of the patient.
Male breasts lack developed lobules; therefore, the majority of the breast cancer type is the invasive ductal carcinoma, which accounts for approximately 90% of the total breast cancers diagnosed in men. The male breast cancer presents typically at later stages and progressed states due to lack of awareness, lack of screening protocols, and the myth that breast cancers affect women alone. The evaluation and workup of male breast cancer is similar to that carried out in women and involves histological evaluation, diagnostic imaging, and evaluation of axillary lymph nodes and analysis of hormone receptor types, which later guides the management algorithm. Approximately 15% of men that develop breast cancer have been identified to have a genetic mutation in BRCA genes, and this warrants the need for BRCA genetic counseling and testing among men.
Staging
The staging of breast cancer in women and men helps in classifying the prognosis as well as determining the treatment guidelines. The TNM staging is typically used in the staging of the breast cancers and is based on the size and invasiveness of the tumor (T), the involvement of regional lymph nodes (N), and the presence or absence of metastatic disease (M).
Stage I of breast cancer involves a tumor with a size of ≤ 20mm (T1), no lymph node (N0), and no metastatic disease (M0).
Stage II of breast cancer involves tumors with a size of > 20mm (T2-3) and/or involvement of 1-3 lymph nodes (N1).
Stage III cancer involves tumors of any size involving the chest wall or skin (T4), and/or involvement of ≥ 4 lymph nodes, or palpable nodes (N2-3).
Stage IV involves any metastatic disease.
Treatment
The main treatment modalities of breast cancer include chemotherapy, surgery, radiation therapy, and hormonal therapy. The most important prognostic factor for breast cancer is the involvement of the axillary lymph nodes and is essential in determining the choice of treatment a patient receives. Before 1994, accurate staging of breast cancer involved complete removal of axillary lymph nodes and axillary lymph node dissection (ALND). However, the procedure was associated with significant morbidity, including lymphedema and nerve damage.
In terms of surgical approach, the axillary lymph nodes are divided into three groups with respect to the pectoralis minor muscle where Level I nodes lie lateral to the pectoralis minor; Level II nodes are found posterior to the pectoralis minor, while Level III nodes lie medial to the pectoralis minor. Historically, axillary lymph node dissection (ALND) often involved the removal of all the axillary group nodes, which meant that the pectoralis minor had to be dissected and divided. The current regimes of therapy used in the management of breast cancer, however, reduce morbidity significantly by limiting the removal of Level I and II nodes while achieving good survival rates.
Sentinel lymph node biopsy
Sentinel lymph node biopsy is based on the concept that the presence of malignant cells in the sentinel node is a reflection of the status of tumor involvement in the entire lymph node region and therefore reduced the need for ALND and the significant morbidity associated with it. Sentinel node biopsy is used with other malignancy types, for example, melanoma. This procedure involves localization of the sentinel lymph node pre-operatively by injection of radiolabeled colloid or blue dye around the affected breast. This helps in the identification of the sentinel lymph node by utilizing a gamma probe or through visual confirmation or sometimes a combination of the two techniques.
Upon identification of the sentinel lymph node, it is removed by the surgeon and sent for examination by a pathologist while the patient remains under anesthesia, awaiting the next procedure. The procedure may require that more than one sentinel lymph node is removed for absolute confirmatory purposes. Negative reports from the pathologist for malignant cells indicate that the cancer is contained within the breast, and therefore, ALND would be unwarranted. If the pathologists indicates positive results for the malignant cells, then the surgeon may need to remove the remainder of the nodes in the axillary region. Sentinel lymph node biopsy has proven successful in the identification of sentinel lymph nodes in approximately 975 of the cases and stands at more than 95% of the time in accurately predicting the status of the remaining axillary lymph nodes.
Adjuvant therapy
In most circumstances, systemic treatment is indicated and commonly involves hormone therapy, chemotherapy, targeted therapy, and radiation. Prior to surgery, neoadjuvant chemotherapy may be utilized to help in the reduction of the tumor burden and the likelihood that the entire tumor may be removed. Radiation therapy is indicated for early-stage, DCIS, and locally advanced forms of breast cancer.
Hormone therapy is another form of breast cancer treatment modality, which relies on the status of hormone receptors on the cancerous cells. To reduce the risk of recurrence and new malignancies, hormone therapy is advised for the entirety of the patient’s life. Targeted therapy, unlike chemotherapy, which does not spare healthy cells, is a novel pharmaceutical treatment that specifically targets a particular type or subtype of tumors.
Lymphedema
Lymphedema is a condition of localized fluid retention and tissue swelling caused by a compromised lymphatic system. Accumulation of interstitial fluid in the downstream extremities is a direct cause of the disruptive nature of cases ALND and sentinel lymph node biopsy have on the lymphatic vessels. Lymphedema is different from the edema caused by venous insufficiency or obstruction due to the fact that lymphedema is naturally low-output failure and the fact that the onset is more insidious and symptoms are less severe.
It is estimated that 20% experience lymphedema following an ALND procedure. A number of factors play a role in the augmentation of the risk and the severity of the condition and include radiation therapy and other accompanying breast cancer treatments, which may increase the risk of lymphedema. There is a reported increase in the risk of venous thrombosis linked to surgery, indwelling venous catheters, and malignancy. Chemotherapy is also associated with cachexia as well as interstitial fluid retention caused by hypoalbuminemia. Obesity, just like in other conditions, also predisposes cancer survivors to increased risk for severe lymphedema.
The persistence of lymphedema can directly lead to subcutaneous fibro-adipose, and dermal tissue accumulation where the skin dries up becomes firm and hyperkeratotic, leading to the development of vesicular skin lesions sometimes. Peau d’orange skin (thick, pock-marked skin like an orange rind) and the Stemmer sign (the inability to tent the skin at the base of the finger or toe) are identified as the hallmarks of lymphedema. The swelling in these patients causes paresthesia, pain, limited range of motion as well as numbness which impairs the normal functioning. The changes in appearance have the ability to drastically reduce the quality of life of a patient.
It is a fact that lymphedema is incurable, at least according to certain literature. However, management has been shown to be worthwhile and possible with the use of complex decongestive physiotherapy. The approach is labor-intensive and involves the use of creams in specific massage techniques, skincare, and use of specific and carefully fitted elastic garments used for compression. Correct application of the complex decongestive physiotherapy delays the fibrosis development increases lymphatic transport and therefore improves lymphedema symptoms.
About the Author:
Based in Perth, Western Australia, Christine Comans is a Clinical Aesthetician, trainer and educator who specialises in intra dermal micro-pigmentation for women after mastectomy and breast reconstruction surgery. Chris is a strong advocate for industry standards and is renowned for her love of learning and sharing her knowledge.
Note from the Author: Sending you my heartfelt thanks for taking the time to read my blog. Over the last year I have been studying the lymphatic system and Manual Lymphatic Drainage (MLD) as part of my degree at the Australasian College of Health and Wellness. Recently I had the pleasure of performing the role of Clinical Supervisor for other degree students studying at the college. My role of clinical supervisor involved teaching a small group of outstanding professionals how to successfully perform the practical application of Manual Lymphatic Drainage MLD. Personally, I believe that MLD is a very underrated modality and now it’s my desire to preach the benefits to all.





12 Responses